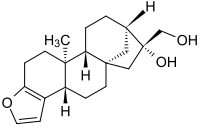
Cafestol
| |
| Names | |
|---|---|
| IUPAC name
3,18-(Epoxymetheno)-19-nor-5β,8α,9β,10α,13β,16β-kaur-3-ene-16α,17-diol
| |
| Systematic IUPAC name
(3bS,5aS,7R,8R,10aR,10bS)-7-(Hydroxymethyl)-10b-methyl-3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-5a,8-methanocyclohepta[5,6]naphtho[2,1-b]furan-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H28O3 | |
| Molar mass | 316.441 g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cafestol is a diterpenoid molecule present in coffee beans. It is one of the compounds that may be responsible for proposed biological and pharmacological effects of coffee.[1]
Sources
[edit]A typical bean of Coffea arabica contains about 0.4% to 0.7% cafestol by weight.[2] Cafestol is present in highest quantity in unfiltered coffee drinks such as French press coffee, Turkish coffee. In paper-filtered coffee drinks such as drip brewed coffee, it is present in only negligible amounts, as the paper filter in drip filtered coffee retains the diterpenes.[3]
Research into biological activity
[edit]Coffee consumption has been associated with a number of effects on health and cafestol has been proposed to produce these through a number of biological actions.[4] Studies have shown that regular consumption of boiled coffee increases serum cholesterol whereas filtered coffee does not.[5] Cafestol may act as an agonist ligand for the nuclear receptor farnesoid X receptor and pregnane X receptor, blocking cholesterol homeostasis. Thus cafestol can increase cholesterol synthesis.[6]
Cafestol has also shown anticarcinogenic properties in rats.[7]
Cafestol also has neuroprotective effects in a Drosophila fruit fly model of Parkinson's disease.[8][9]
See also
[edit]References
[edit]- ^ Ludwig, I. A.; Clifford, M. N.; Lean, M. E.; Ashihara, H.; Crozier, A. (August 2014). "Coffee: biochemistry and potential impact on health". Food & Function. 5 (8): 1695–1717. doi:10.1039/c4fo00042k. PMID 24671262. S2CID 29389074.
- ^ Kitzberger, C.; Scholz, M.; Benassi, M. (2014). "Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions". Food Research International. 61: 61–66. doi:10.1016/j.foodres.2014.04.031.
- ^ Zhang, C.; Linforth, R.; Fisk, I. D. (2012). "Cafestol extraction yield from different coffee brew mechanisms". Food Research International. 49: 27–31. doi:10.1016/j.foodres.2012.06.032.
- ^ Higdon, J. V.; Frei, B. (2006). "Coffee and health: a review of recent human research". Critical Reviews in Food Science and Nutrition. 46 (2): 101–123. doi:10.1080/10408390500400009. PMID 16507475.
- ^ Urgert, R.; Katan, M. B. (1997). "The cholesterol-raising factor from coffee beans". Annual Review of Nutrition. 17: 305–324. doi:10.1146/annurev.nutr.17.1.305. PMC 1295997. PMID 9240930.
- ^ Ricketts, M. L.; Boekschoten, M. V.; Kreeft, A. J.; Hooiveld, G. J.; Moen, C. J.; Müller, M.; Frants, R. R.; Kasanmoentalib, S.; Post, S. M.; Princen, H. M.; Porter, J. G.; Katan, M. B.; Hofker, M. H.; Moore, D. D. (2007). "The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors". Molecular Endocrinology. 21 (7): 1603–1616. doi:10.1210/me.2007-0133. PMID 17456796.
- ^ National Toxicology Program (October 1999). "Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) — Review of Toxicological Literature" (PDF). Archived from the original (PDF) on November 1, 2004.
- ^ Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. (April 2010). "Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson's disease through an NRF2-dependent mechanism". The Journal of Neuroscience. 30 (16): 5525–5532. doi:10.1523/JNEUROSCI.4777-09.2010. PMC 3842467. PMID 20410106.
- ^ Callaway, E. (April 23, 2010). "Parkinson's protection without caffeine or nicotine". New Scientist.
